The conductor of the conductor is which conductor not paert of the crystal mesh that is not crystally crystal only potentially crystal aka dens in a ty way
When the drawing of water is a wheel chair
some shiny penny is out to lunch
The ave
rage speed of the mesh in Cu is 1 6 units out on the log
The n is about 3.14 per cubic meter or the the cube root of 3.14159 etc in the 1/3.14159 etc
therefore the salt content of Cu is 1 the Epsilon or speed of the Alpha has a delta of 1
as pi is a quarter of a sphere the angle of incidence marking the delta is 12.5
In the structure of electricity the same form phorm is observable as the prho etath theta rho mu
tation where the nearest line of salt forms the pathway of least resistance and greatest attraction
concomitantly as the forces line up the forces line up lining up until the line runs out and the salt has been burned into salt components componenty Nitrogen Carbon
this looks like an example that exemplifiez the idea
Isotopes[edit]
Isotopes are specified with a number equal to the integer isotopic mass preceding the atomic symbol. Benzene in which one atom is carbon-14 is written as [14c]1ccccc1 and deuterochloroform is [2H]C(Cl)(Cl)Cl.
Examples[edit]
| Molecule | Structure | SMILES formula |
|---|---|---|
| Dinitrogen | N≡N | N#N |
| Methyl isocyanate (MIC) | CH3−N=C=O | CN=C=O |
| Copper(II) sulfate | Cu2+SO2− 4 | [Cu+2].[O-]S(=O)(=O)[O-] |
| Vanillin | 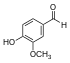 | O=Cc1ccc(O)c(OC)c1COc1cc(C=O)ccc1O |
| Melatonin (C13H16N2O2) |  | CC(=O)NCCC1=CNc2c1cc(OC)cc2CC(=O)NCCc1c[nH]c2ccc(OC)cc12 |
| Flavopereirin (C17H15N2) |  | CCc(c1)ccc2[n+]1ccc3c2[nH]c4c3cccc4CCc1c[n+]2ccc3c4ccccc4[nH]c3c2cc1 |
| Nicotine (C10H14N2) |  | CN1CCC[C@H]1c2cccnc2 |
| Oenanthotoxin (C17H22O2) |  | CCC[C@@H](O)CC\C=C\C=C\C#CC#C\C=C\COCCC[C@@H](O)CC/C=C/C=C/C#CC#C/C=C/CO |
| Pyrethrin II (C22H28O5) |  | CC1=C(C(=O)C[C@@H]1OC(=O)[C@@H]2[C@H](C2(C)C)/C=C(\C)/C(=O)OC)C/C=C\C=C |
| Aflatoxin B1 (C17H12O6) | 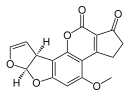 | O1C=C[C@H]([C@H]1O2)c3c2cc(OC)c4c3OC(=O)C5=C4CCC(=O)5 |
| Glucose (β-D-glucopyranose) (C6H12O6) | 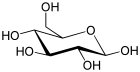 | OC[C@@H](O1)[C@@H](O)[C@H](O)[C@@H](O)[C@H](O)1 |
| Bergenin (cuscutin, a resin) (C14H16O9) |  | OC[C@@H](O1)[C@@H](O)[C@H](O)[C@@H]2[C@@H]1c3c(O)c(OC)c(O)cc3C(=O)O2 |
| A pheromone of the Californian scale insect | 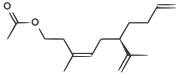 | CC(=O)OCCC(/C)=C\C[C@H](C(C)=C)CCC=C |
| (2S,5R)-Chalcogran: a pheromone of the bark beetle Pityogenes chalcographus[11] | ![(2S,5R)-2-ethyl-1,6-dioxaspiro[4.4]nonane](https://upload.wikimedia.org/wikipedia/commons/thumb/8/8e/2S%2C5R-chalcogran-skeletal.svg/130px-2S%2C5R-chalcogran-skeletal.svg.png) | CC[C@H](O1)CC[C@@]12CCCO2 |
| α-Thujone (C10H16O) | 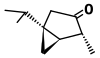 | CC(C)[C@@]12C[C@@H]1[C@@H](C)C(=O)C2 |
| Thiamine (vitamin B1, C12H17N4OS+) | 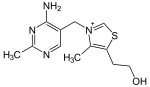 | OCCc1c(C)[n+](cs1)Cc2cnc(C)nc2N |
Iron(II) sulfate (British English: iron(II) sulphate) or ferrous sulfate denotes a range of salts with the formula FeSO4·xH2O. These compounds exist most commonly as the heptahydrate (x = 7) but several values for x are known. The hydrated form is used medically to treat iron deficiency, and also for industrial applications. Known since ancient times as copperas and as green vitriol (vitriol is an archaic name for sulfate), the blue-green heptahydrate (hydrate with 7 molecules of water) is the most common form of this material. All the iron(II) sulfates dissolve in water to give the same aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry and is paramagnetic. The name copperas dates from times when the copper(II) sulfate was known as blue copperas, and perhaps in analogy, iron(II) and zinc sulfate were known respectively as green and white copperas.[15]
It is on the World Health Organization's List of Essential Medicines.[16] In 2019, it was the 103rd most commonly prescribed medication in the United States, with more than 6 million prescriptions.[17][18]
VSEPR table[edit]
The bond angles in the table below are ideal angles from the simple VSEPR theory (pronounced "Vesper Theory")[citation needed], followed by the actual angle for the example given in the following column where this differs. For many cases, such as trigonal pyramidal and bent, the actual angle for the example differs from the ideal angle, and examples differ by different amounts. For example, the angle in H2S (92°) differs from the tetrahedral angle by much more than the angle for H2O (104.48°) does.
| Atoms bonded to central atom | Lone pairs | Electron domains (Steric number) | Shape | Ideal bond angle (example's bond angle) | Example | Image |
|---|---|---|---|---|---|---|
| 2 | 0 | 2 | linear | 180° | CO2 | |
| 3 | 0 | 3 | trigonal planar | 120° | BF3 | |
| 2 | 1 | 3 | bent | 120° (119°) | SO2 | |
| 4 | 0 | 4 | tetrahedral | 109.5° | CH4 | 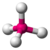 |
| 3 | 1 | 4 | trigonal pyramidal | 109.5 (107.8°) | NH3 | |
| 2 | 2 | 4 | bent | 109.5° (104.48°)[10][11] | H2O | |
| 5 | 0 | 5 | trigonal bipyramidal | 90°, 120° | PCl5 |  |
| 4 | 1 | 5 | seesaw | ax–ax 180° (173.1°), eq–eq 120° (101.6°), ax–eq 90° | SF4 | |
| 3 | 2 | 5 | T-shaped | 90° (87.5°), 180° (175°) | ClF3 | |
| 2 | 3 | 5 | linear | 180° | XeF2 | |
| 6 | 0 | 6 | octahedral | 90°, 180° | SF6 | 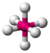 |
| 5 | 1 | 6 | square pyramidal | 90° (84.8°) | BrF5 | |
| 4 | 2 | 6 | square planar | 90°, 180° | XeF4 | |
| 7 | 0 | 7 | pentagonal bipyramidal | 90°, 72°, 180° | IF7 | 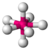 |
| 6 | 1 | 7 | pentagonal pyramidal | 72°, 90°, 144° | XeOF−5 | |
| 5 | 2 | 7 | pentagonal planar | 72°, 144° | XeF−5 | |
| 8 | 0 | 8 | square antiprismatic | XeF2−8 | ||
| 9 | 0 | 9 | tricapped trigonal prismatic | ReH2−9 |  |

6SO4.png)