KloΖα ζ ΑΗχαή AKA Γκ Ζα ζΑ ζ Ζ ζ α Α α
Ελλεκτρικιτυ Ελεκτρίζίζ Electricity
ASS U sSseethe diregject ly below the shiny pen[][] dszZz {ARE} se(m)lling (U) roundlyas they say in the queenssssssssssThis particular article issssssbout chemical SsubsStancessssdanger will robinsonsee ell els warefor more adsssS! Sodium chloride
 Sodium chloride crystals in a form of halite | |
 Crystal structure with sodium in purple and chloride in green[1] | |
| Names | |
|---|---|
| IUPAC name Sodium chloride | |
Other names
| |
| Identifiers | |
3D model (JSmol) | |
| 3534976 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.028.726 |
| EC Number |
|
| 13673 | |
| KEGG | |
| MeSH | Sodium+chloride |
PubChem CID | |
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) | |
| Properties | |
| NaCl | |
| Molar mass | 58.443 g/mol[2] |
| Appearance | Colorless cubic crystals[2] |
| Odor | Odorless |
| Density | 2.17 g/cm3[2] |
| Melting point | 800.7 °C (1,473.3 °F; 1,073.8 K)[2] |
| Boiling point | 1,465 °C (2,669 °F; 1,738 K)[2] |
| 360 g/1000 g pure water at T = 25 °C[2] | |
| Solubility in ammonia | 21.5 g/L at T = ?[clarification needed] |
| Solubility in methanol | 14.9 g/L at T = ?[clarification needed] |
| −30.2·10−6 cm3/mol[3] | |
Refractive index (nD) | 1.5441 (at 589 nm)[4] |
| Structure[5] | |
| Face-centered cubic (see text), cF8 | |
| Fm3m (No. 225) | |
| a = 564.02 pm | |
Formula units (Z) | 4 |
| octahedral at Na+ octahedral at Cl− | |
| Thermochemistry[6] | |
Heat capacity (C) | 50.5 J/(K·mol) |
Std molar entropy (S | 72.10 J/(K·mol) |
Std enthalpy of formation (ΔfH⦵298) | −411.120 kJ/mol |
| Pharmacology | |
| A12CA01 (WHO) B05CB01 (WHO), B05XA03 (WHO), S01XA03 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 3 g/kg (oral, rats)[7] |
| Related compounds | |
Other anions | Sodium fluoride Sodium bromide Sodium iodide Sodium astatide |
Other cations | Lithium chloride Potassium chloride Rubidium chloride Caesium chloride Francium chloride |
| Supplementary data page | |
| Sodium chloride (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Sodium chloride /ˌsoʊdiəm ˈklɔːraɪd/,[8] commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g Na and 60.66 g Cl. Sodium chloride is the salt most responsible for the salinity of seawater and of the extracellular fluid of many multicellular organisms. In its edible form of table salt, it is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. A second major application of sodium chloride is de-icing of roadways in sub-freezing weather.
Uses[edit source]
In addition to the familiar domestic uses of salt, more dominant applications of the approximately 250 million tonnes per year production (2008 data) include chemicals and de-icing.[9]
Chemicals functions[edit source]
Salt is used, directly or indirectly, in the production of many chemicals, which consume most of the world's production.[10]
Chlor-alkali industry[edit source]
It is the starting point for the chloralkali process, the industrial process to produce chlorine and sodium hydroxide, according to the chemical equation
- 2 NaCl + 2 H2O electrolysis→ Cl2 + H2 + 2 NaOH
This electrolysis is conducted in either a mercury cell, a diaphragm cell, or a membrane cell. Each of those uses a different method to separate the chlorine from the sodium hydroxide. Other technologies are under development due to the high energy consumption of the electrolysis, whereby small improvements in the efficiency can have large economic paybacks. Some applications of chlorine include PVC thermoplastics production, disinfectants, and solvents.
Sodium hydroxide is extensively used in many different inustries enabling production of paper, soap, and aluminium etc.
Soda-ash industry[edit source]
Sodium chloride is used in the Solvay process to produce sodium carbonate and calcium chloride. Sodium carbonate, in turn, is used to produce glass, sodium bicarbonate, and dyes, as well as a myriad of other chemicals. In the Mannheim process, sodium chloride is used for the production of sodium sulfate and hydrochloric acid.
Standard[edit source]
Sodium chloride has an international standard that is created by ASTM International. The standard is named ASTM E534-13 and is the standard test methods for chemical analysis of sodium chloride. These methods listed provide procedures for analyzing sodium chloride to determine whether it is suitable for its intended use and application.
Miscellaneous industrial uses[edit source]
Sodium chloride is heavily used, so even relatively minor applications can consume massive quantities. In oil and gas exploration, salt is an important component of drilling fluids in well drilling. It is used to flocculate and increase the density of the drilling fluid to overcome high downwell gas pressures. Whenever a drill hits a salt formation, salt is added to the drilling fluid to saturate the solution in order to minimize the dissolution within the salt stratum.[9] Salt is also used to increase the curing of concrete in cemented casings.[10]
In textiles and dyeing, salt is used as a brine rinse to separate organic contaminants, to promote "salting out" of dyestuff precipitates, and to blend with concentrated dyes to standardize[clarification needed] them. One of its main roles is to provide the positive ion charge to promote the absorption of negatively charged ions of dyes.[10]
It is also used in processing aluminium, beryllium, copper, steel and vanadium. In the pulp and paper industry, salt is used to bleach wood pulp. It also is used to make sodium chlorate, which is added along with sulfuric acid and water to manufacture chlorine dioxide, an excellent oxygen-based bleaching chemical. The chlorine dioxide process, which originated in Germany after World War I, is becoming more popular because of environmental pressures to reduce or eliminate chlorinated bleaching compounds. In tanning and leather treatment, salt is added to animal hides to inhibit microbial activity on the underside of the hides and to attract moisture back into the hides.[10]
In rubber manufacture, salt is used to make buna, neoprene and white rubber types. Salt brine and sulfuric acid are used to coagulate an emulsified latex made from chlorinated butadiene.[10][9]
Salt also is added to secure the soil and to provide firmness to the foundation on which highways are built. The salt acts to minimize the effects of shifting caused in the subsurface by changes in humidity and traffic load.[10]
Sodium chloride is sometimes used as a cheap and safe desiccant because of its hygroscopic properties, making salting an effective method of food preservation historically; the salt draws water out of bacteria through osmotic pressure, keeping it from reproducing, a major source of food spoilage. Even though more effective desiccants are available, few are safe for humans to ingest.
Water softening[edit source]
Hard water contains calcium and magnesium ions that interfere with action of soap and contribute to the buildup of a scale or film of alkaline mineral deposits in household and industrial equipment and pipes. Commercial and residential water-softening units use ion-exchange resins to remove ions that cause the hardness. These resins are generated and regenerated using sodium chloride.[10][9]
Road salt[edit source]
The second major application of salt is for de-icing and anti-icing of roads, both in grit bins and spread by winter service vehicles. In anticipation of snowfall, roads are optimally "anti-iced" with brine (concentrated solution of salt in water), which prevents bonding between the snow-ice and the road surface. This procedure obviates the heavy use of salt after the snowfall. For de-icing, mixtures of brine and salt are used, sometimes with additional agents such as calcium chloride and/or magnesium chloride. The use of salt or brine becomes ineffective below −10 °C (14 °F).
Salt for de-icing in the United Kingdom predominantly comes from a single mine in Winsford in Cheshire. Prior to distribution it is mixed with <100 ppm of sodium ferrocyanide as an anti-caking agent, which enables rock salt to flow freely out of the gritting vehicles despite being stockpiled prior to use. In recent years this additive has also been used in table salt. Other additives had been used in road salt to reduce the total costs. For example, in the US, a byproduct carbohydrate solution from sugar-beet processing was mixed with rock salt and adhered to road surfaces about 40% better than loose rock salt alone. Because it stayed on the road longer, the treatment did not have to be repeated several times, saving time and money.[10]
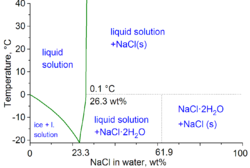
In the technical terms of physical chemistry, the minimum freezing point of a water-salt mixture is −21.12 °C (−6.02 °F) for 23.31 wt% of salt. Freezing near this concentration is however so slow that the eutectic point of −22.4 °C (−8.3 °F) can be reached with about 25 wt% of salt.[11]